Talc, Silica, Sulfates
Talc
Talc has been used since recorded time, mainly for its absorbent properties.
In cosmetics in modifies the feel, makes the product opaque, and keeps it from drying out.
Talc is a hydrous magnesium silicate with a chemical formula of Mg3Si4O10(OH)2
The controversy about talc involves its possible contamination with asbestos, which is found in close proximity to where talc is mined.
The U.S. Food and Drug Administration is actively checking products containing talc to see if any are contaminated with asbestos. They only test 50 samples per year, so don’t depend on the FDA to tell you if a talc product is contaminated with asbestos.
Stick antiperspirant containing talc is not a problem because of the talc. It’s the aluminum salt that plugs up sweat glands in a mistaken notion that stopping perspiration is healthy.
Silica
Potential Health Benefits of Silica
A soluble form of silicon, orthosilicic acid is the form predominantly absorbed by animals and carries the silicon atom to tendons, heart, liver, kidney, and bones. In bones, silicon aids the deposition of calcium in both initial bone growth and repair of damaged bone.
Aluminum is toxic at the cellular level. There is a unique affinity between aluminum and silicon, which may function to reduce the absorption of aluminum.
Silica is an important micro-nutrient but when advertisers create fear around a common ingredient, people mindlessly believe that silica is evil. It’s not, but we don’t use it in deodorme because it’s unnecessary.
Sulfates
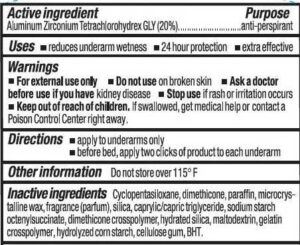 The world’s #1 manufacturer of antiperspirant deodorant does have sulfates in their formula. It’s not because sulfates have a bad reputation. Sulfates are in products that strip off oils and wash away. Antiperspirant is designed to stop perspiration and remain in place for a long time.
The world’s #1 manufacturer of antiperspirant deodorant does have sulfates in their formula. It’s not because sulfates have a bad reputation. Sulfates are in products that strip off oils and wash away. Antiperspirant is designed to stop perspiration and remain in place for a long time.
What are sulfates and why do they get such bad press. Maybe it’s because they are synthetic, which is another bad thing. Almost unnatural.
Sulfates are just any of thousands of chemicals that contain sulfur, an important mineral to maintain life. Sodium sulfate is found in natural spring water. But that’s not the sulfates that have the bad reputation.
Detergents
Ammonium laureth sulfate (ALS) and sodium lauryl sulfate (SLS) are two common surfactants (surface-acting agents) used in soaps and shampoos.
One end of the molecule attaches to water, the other end easily attaches to fat or oils. That’s the way they strip off substances that don’t easily dissolve in water.
They are the detergents found in shampoos, dish soap, body wash, toothpaste, hand soap, laundry detergent, and some cosmetics.
They get the bad reputation because they are used in excess and are irritating to skin. They remove oil from the skin that is part of the skin’s barrier function. Disrupting the skin also disrupts the bacteria residing on the skin.